

Historically the Debye was defined in terms of the dipole moment resulting from two equal charges of opposite sign and separated by 1 Ångstrom (10 -10 m) as 4.801 D. This is thought to produce values too large to be practical on the molecular scale so bond dipole moments are commonly measured in Debye, represented by the symbol, D. The SI unit for an electric dipole moment is the coulomb-meter, (C m).
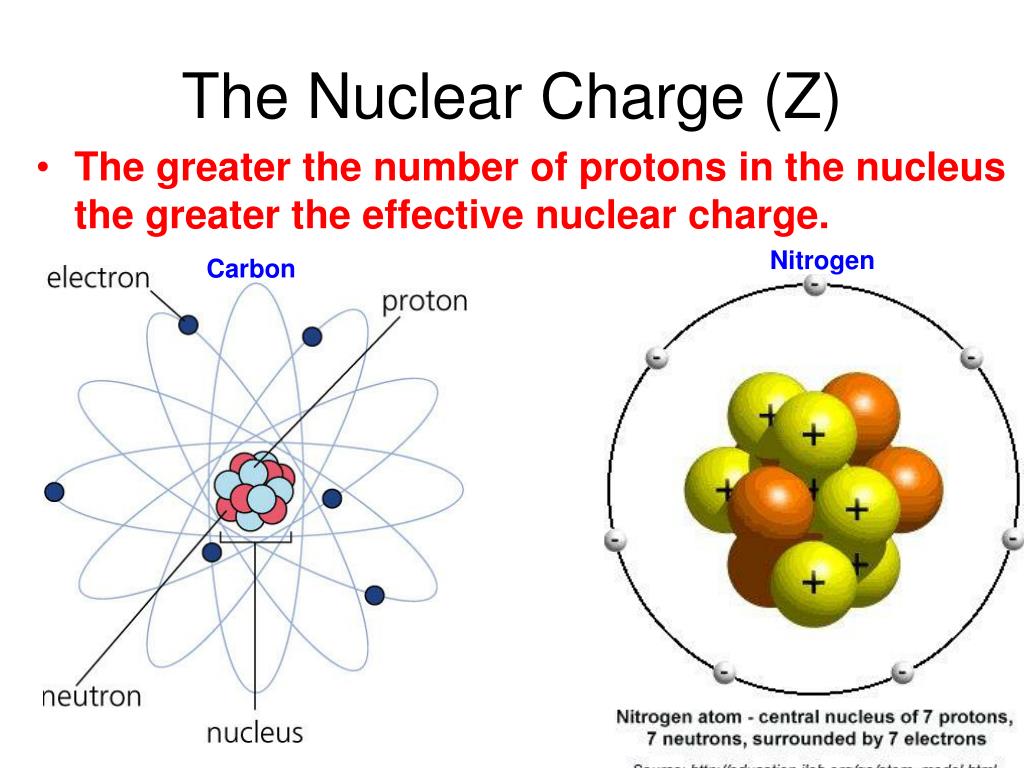
#Effective nuclear charge of oxygen plus
It is a vector, parallel to the bond axis and by convention points from minus to plus (note that many texts appear to ignore the convention and point from plus to minus). The bond dipole μ is given by:Ī bond dipole is modeled as +δ - δ- with a distance d between the partial charges. It occurs whenever there is a separation of positive and negative charges. The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The percentage of ionic character in a compound can be estimated from dipole moments. Percentage of ionic character and charge distributionīased on Fajan's rules, it is expected that every ionic compound will have at least some amount of covalent character. It is found that the greater the possibility of polarization, the lower is the melting point and heat of sublimation and the greater is the solubility in non-polar solvents. Thus zinc (II) chloride ( Zn(II) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 and Cl - 1s 2 2s 2 2p 6 3s 2 3p 6 ) is more covalent than magnesium chloride ( Mg(II) 1s 2 2s 2 2p 6) despite the Zn 2 + ion (74 pm) and Mg 2 + ion (72 pm) having similar sizes and charges.įrom an MO perspective, the orbital overlap disperses the charge on each ion and so weakens the electrovalent forces throughout the solid, this can be used to explain the trend seen for the melting points of lithium halides. < < and < < Įlectronic configuration of the cation: for two cations of the same size and charge, the one with a pseudo noble-gas configuration (with 18 electrons in the outer-most shell) will be more polarizing than that with a noble gas configuration (with 8 electrons in the outermost shell). The cation charge increases (size decreases) and on the right, the anion size increases, both variations leading to an increase in the covalency. The greater the positive charge, the smaller the cation becomes and the ionic potential is a measure of the charge to radius ratio. Large cations are to be found on the bottom left of the periodic table and small anions on the top right. Large charges: as the charge on an ion increases, the electrostatic attractions of the cation for the outer electrons of the anion increases, resulting in the degree of covalent bond formation increasing.This explains why for the common halides, iodides, are the most covalent in nature (I - 206 pm). Large anion: the high polarizability stems from the larger size where the outer electrons are more loosely held and can be more easily distorted by the cation.This explains why LiBr is more covalent than KBr (Li + 90 pm cf. Small cation: the high polarizing power stems from the greater concentration of positive charge on a small area.The polarizing power and polarizability that enhances the formation of covalent bonds is favoured by the following factors: The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by the cation is known as its polarizability. If the degree of polarization is quite small, an ionic bond is formed, while if the degree of polarization is large, a covalent bond results. This results in a distortion, deformation or polarization of the anion. When two oppositely charged ions (X + and Y -) approach each other, the cation attracts electrons in the outermost shell of the anion but repels the positively charged nucleus. \)įajan's rules for predicting whether a bond is predominantly Covalent or Ionic CovalentĪlthough the bond in a compound like X +Y - may be considered to be 100% ionic, it will always have some degree of covalent character.


 0 kommentar(er)
0 kommentar(er)
